PHARMACEUTICAL QUALITY MANAGEMENT SYSTEM
A QMS system designed to address the complex
requirements of regulations and standards.
Request a Demo
Trusted by Companies Worldwide





Pharmaceutical Quality Management System Accelerates Time to Market
Life expectancy and quality of life throughout the world is increasing, thanks in large part to innovative drugs. Companies in the pharmaceutical industry and the biotechnology industry must develop new medicines in a timely manner to continue their life-enhancing missions. The stopwatch is ticking every day your product isn’t on the market.
Compliance with current good manufacturing practice (cGMP) regulations is a part of standard pharmaceutical quality operations. However, the use of legacy systems and paper-based processes make it difficult to control processes and systematic oversight.
AssurX’s pharmaceutical quality management system automates of all processes required to ensure that final products are manufactured according to customer and regulatory requirements. A platform of interconnected workflows resolve and prevent quality deviations.
|
|
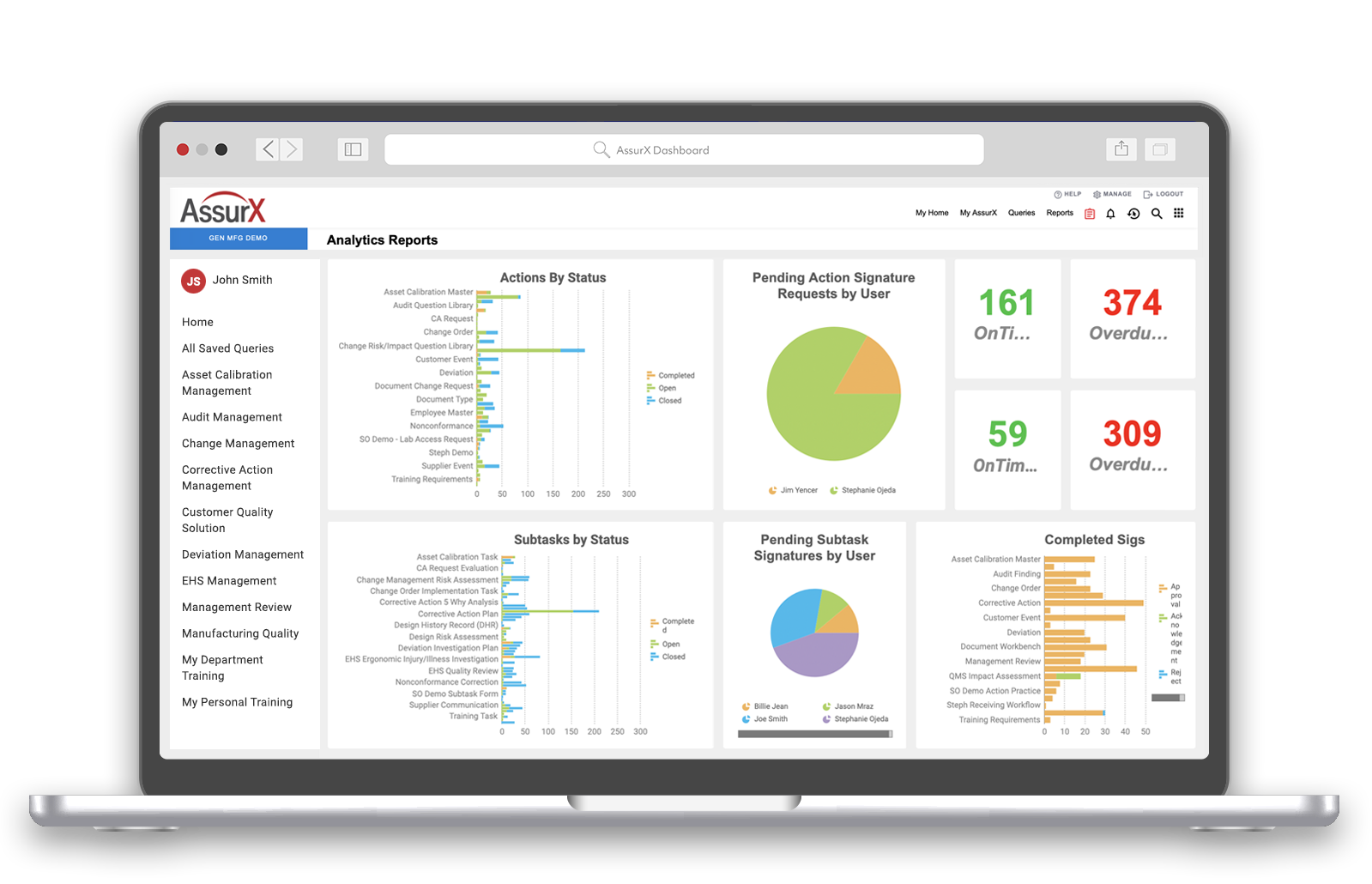
Over the course of a year, EyePoint Pharmaceuticals went from having separate solutions for document management and training to a fully integrated quality management system. Solutions deployed in AssurX include document management, training management, supplier quality, CAPA, internal and external audits.




