MEDICAL DEVICE & DIAGNOSTICS
A harmonized framework for managing medical device quality and regulatory compliance.
Request a Demo
Trusted by Companies Worldwide




The AssurX Medical Device Quality Management System: Aligning Quality Objectives with Compliance Requirements
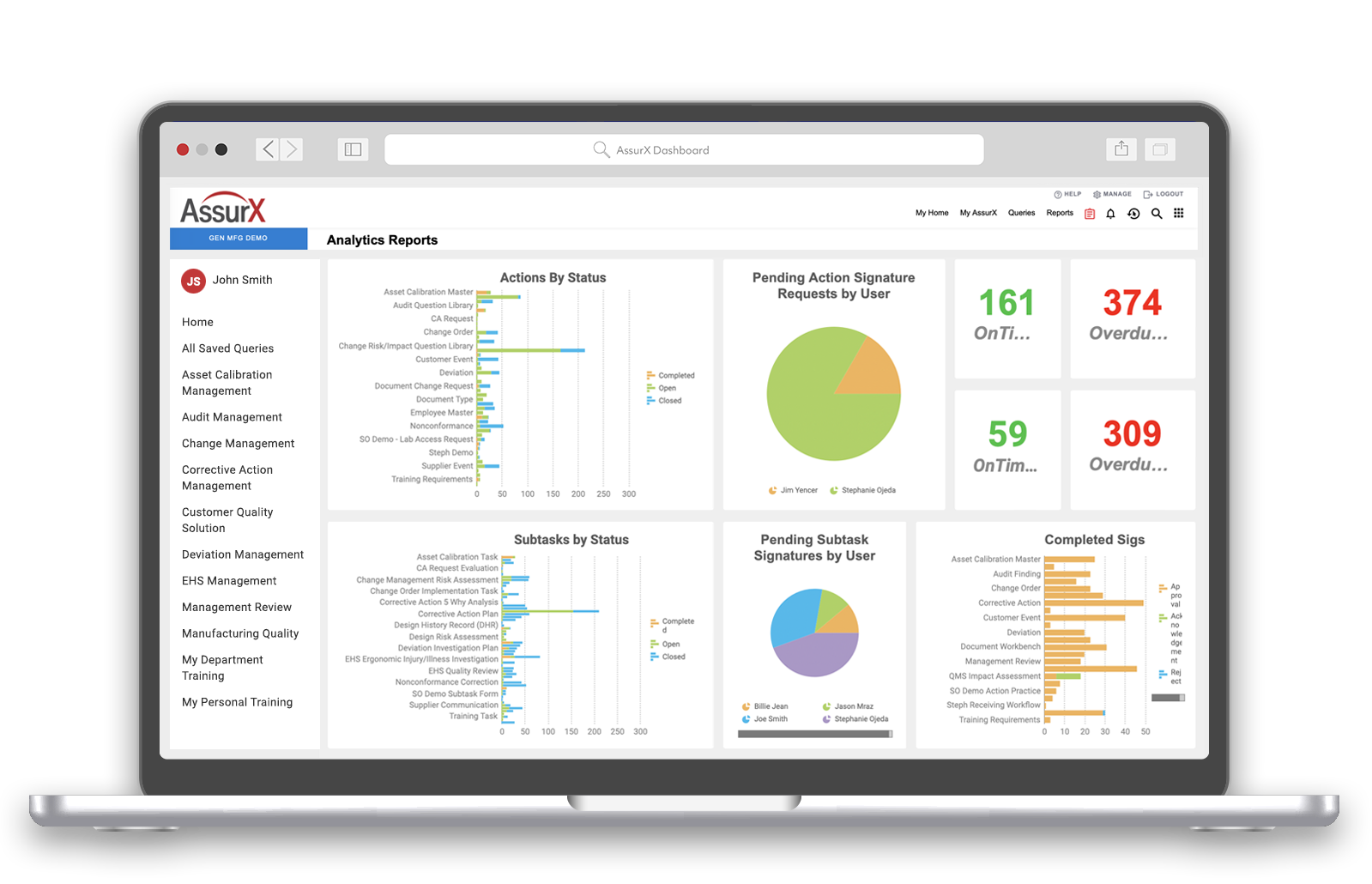
The medical device market is full of opportunities to create life-saving products that diagnose and treat patients worldwide. AssurX medical device quality management system is designed to support manufacturers in marketing safe and effective medical devices. Additionally, it demonstrates compliance to meet those demands.
Device and diagnostics manufacturers use the AssurX EQMS to bridge the gap between aligning quality processes to improve product safety. They also maintain medical device compliance with applicable regulations.
|
|